Eosinophilic oesophagitis
Notes
Overview
Eosinophilic oesophagitis is a chronic immune-mediated disease, characterised by eosinophil-predominant inflammation.
Eosinophilic oesophagitis (EO or EoE if American spelling) is an increasingly recognised oesophageal disorder. It is a chronic immune-mediated oesophageal disorder that is characterised clinically by symptoms of oesophageal dysfunction and histologically by an eosinophil-predominant inflammatory response.
EO usually occurs in the third and fourth decades of life and most commonly affects men with some genetic elements identified.
Aetiology
Eosinophilic oesophagitis is an allergy-driven disease.
Like most allergy-driven diseases, the causes of EO is incompletely understood but related to genetic, environmental and host immune factors.
There is a strong association between EO and other allergy-driven diseases including:
- Asthma
- Atopic dermatitis
- Food allergies
- Environmental allergies
Pathophysiology
Eosinophilic oesophagitis causes a Th2-mediated response with release of cytokines IL-5/IL-13.
Antigens from food, and lesser extent inhaled, drive a Th2-mediated inflammatory response, which leads to the release of cytokines IL-5 and IL-13. These cytokines trigger epithelial cells of the oesophagus to secrete eotaxin.
Eotaxin is able to recruit eosinophils from the peripheral blood to the oesophageal mucosa. Other inflammatory cells typical of an allergy-type response are also recruited to the oesophageal mucosa including mast cells and T helper cells (Th2 are a subset of T helper cells).
Over time, there are cycles of inflammation and remodelling, which leads to narrowing and reduced distensibility of the oesophagus. Unfortunately, the condition will generally relapse on cessation of treatment.
Clinical features
The cardinal symptom of eosinophilic oesophagitis is dysphagia.
There is usually a history of slow eating, requiring lots of water to help with swallowing during meals. One of the characteristic presentations of EO is food bolus (discussed below).
Other features of ‘oesophageal dysfunction’ may be present:
- Heartburn
- Dyspepsia
- Chest pain
Food bolus
Patients with EO are predisposed to obstruction of the oesophagus with a food bolus (often poorly chewed food, usually meat). This often results in presentation to accident and emergency and may require endoscopic removal.
Patients presenting with food bolus should have biopsies taken at the time of endoscopy to exclude EO. Food bolus may occur even in patients with mild symptoms.
Diagnosis
The diagnosis EO is requires histological confirmation of eosinophil-predominant inflammation.
There are specific criteria for the diagnosis of EO based on the International Consensus Diagnostic Criteria:
- Symptoms: related to oesophageal dysfunction
- Eosinophil-predominant inflammation: identified on oesophageal biopsy (e.g. ≥15 eosinophils per high power field)
- Exclude other causes: several conditions are associated with oesophageal eosinophilia
Conditions associated with oesophageal eosinophilia can include gastro-oesophageal reflux (GORD), oesophageal infections, Crohn’s disease (with oesophageal involvement) and connective tissue disease among others. PPI-responsive oesophageal eosinophilia, which originally required exclusion with a trial of PPI prior to biopsy, is now considered a subset of EO.
Investigations
The key investigation is a gastroscopy and biopsy to confirm the presence of eosinophil-predominant inflammation.
Upper GI endoscopy
The endoscopic appearance in EO may be normal, or there may be classic features of EO including linear furrows (longitudinal cracks in the oesophageal mucosa), white spots due to eosinophilic abscesses, schatzki rings (narrowing at the lower oesophagus) or multiple circular rings (termed ‘trachealisation’).
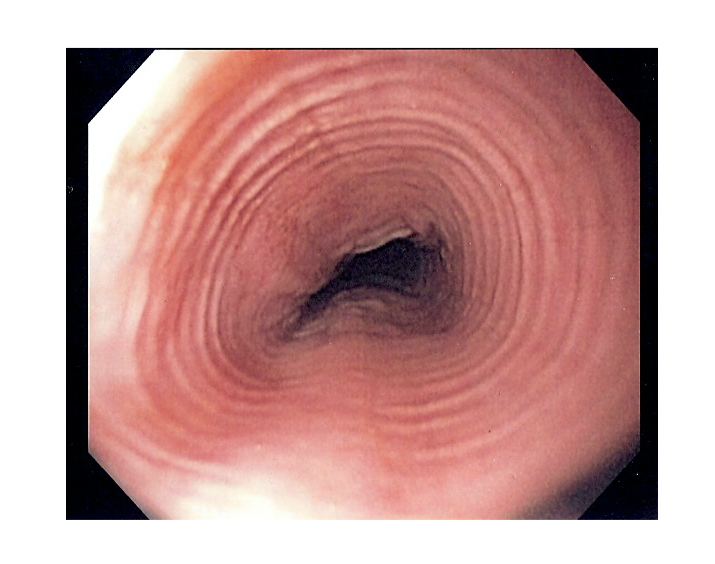
Endoscopic appearance of 'trachealisation' in eosinophilic oesophagitis
Image courtesy of Wikimedia Commons
Others
Additional investigations are guided by the suspected underlying diagnosis. Commonly, EO is associated with elevated levels of IgE and peripheral eosinophilia, but this is usually mild.
Management
Management of EO is aimed at preventing long-term fibrosis and stricture formation.
Management of EO is broadly divided into three categories: conservative, medical and endoscopic intervention.
Conservative
Dietary interventions have been proposed in EO, which include elemental diets and dietary elimination. Advice from a dietitian should always be sought to prevent nutritional deficiencies from an elimination diet that is too restrictive.
Medical
The hallmark of treatment in EO is pharmacological therapy with PPI and topical steroids. As discussed, a subset of patients with EO may have PPI-responsive oesophageal eosinophilia that will improve after starting treatment with PPI.
The mainstay of treatment is topical steroids (e.g. fluticasone/budesonide). Fluticasone is an inhaled preparation whereas budesonide is a viscus slurry. Both have been linked to clinical symptom improvement and histological remission.
Newer biologic therapies that target the cytokine IL-5, which is critical in the pathogenesis, are starting to be used for EO as part of clinical trials.
Endoscopic intervention
The main complication of EO is stricture formation, which refers to a narrowing in the oesophagus. Patients are at increased risk of food bolus, malnutrition and aspiration pneumonia.
Once medical therapy has been optimised, strictures may be treated with oesophageal dilatation. The main complication of dilatation is oesophageal perforation.
Last updated: March 2021
Have comments about these notes? Leave us feedback
