Macrocytic anaemia
Notes
Overview
Macrocytic anaemia describes the presence of a reduced haemoglobin concentration and an increase in the mean corpuscular volume (MCV).
Normal RBC haemoglobin concentration for males is approximately 130-175 g/L and for females approximately 120-155 g/L. A haemoglobin (Hb) concentration below this levels is considered to be anaemic.
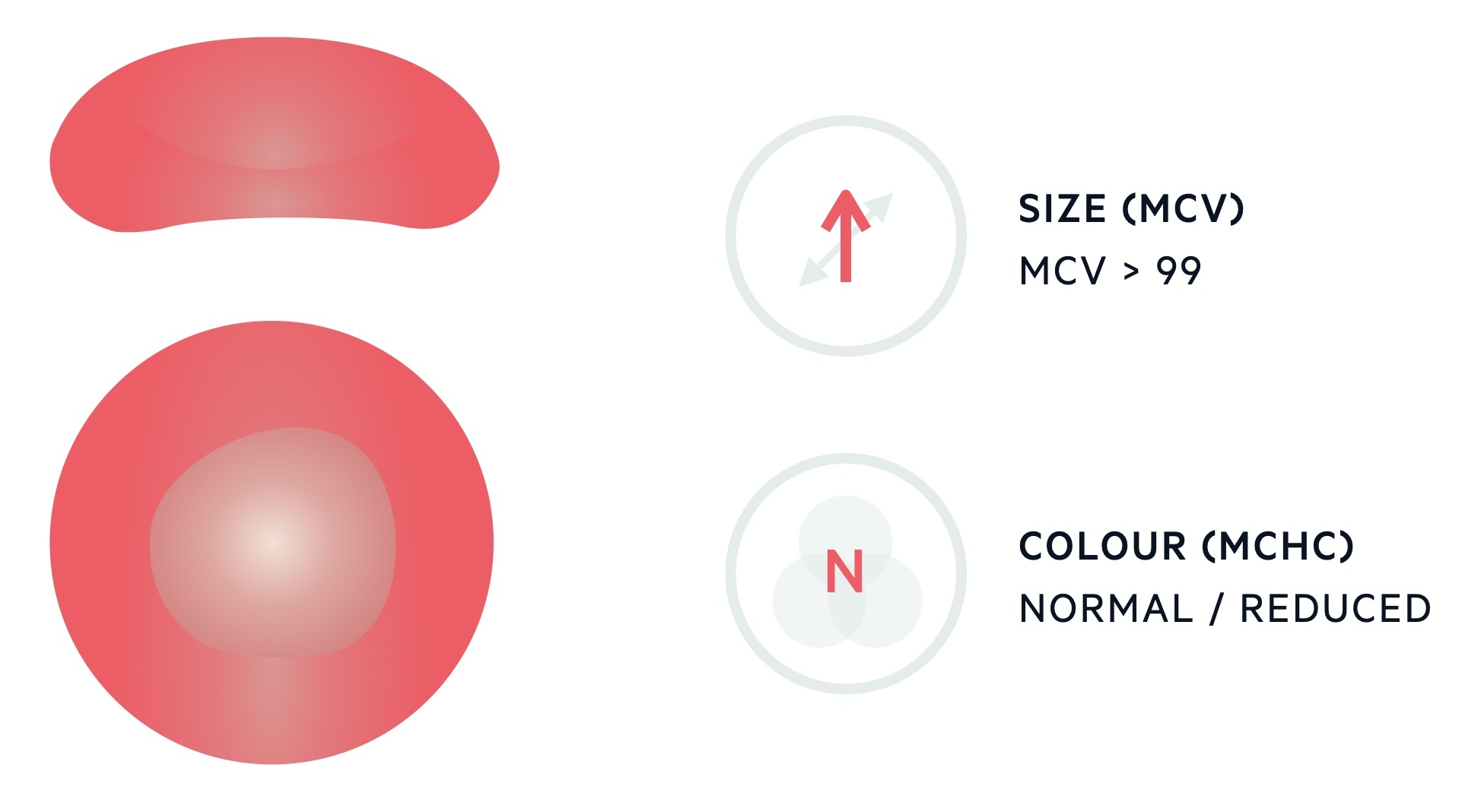
The MCV describes the mean volume of erythrocytes and is measured in femtolitres (fL). The standard range for erythrocytes is 82-99 fL. Levels > 99 fL are considered macrocytic.
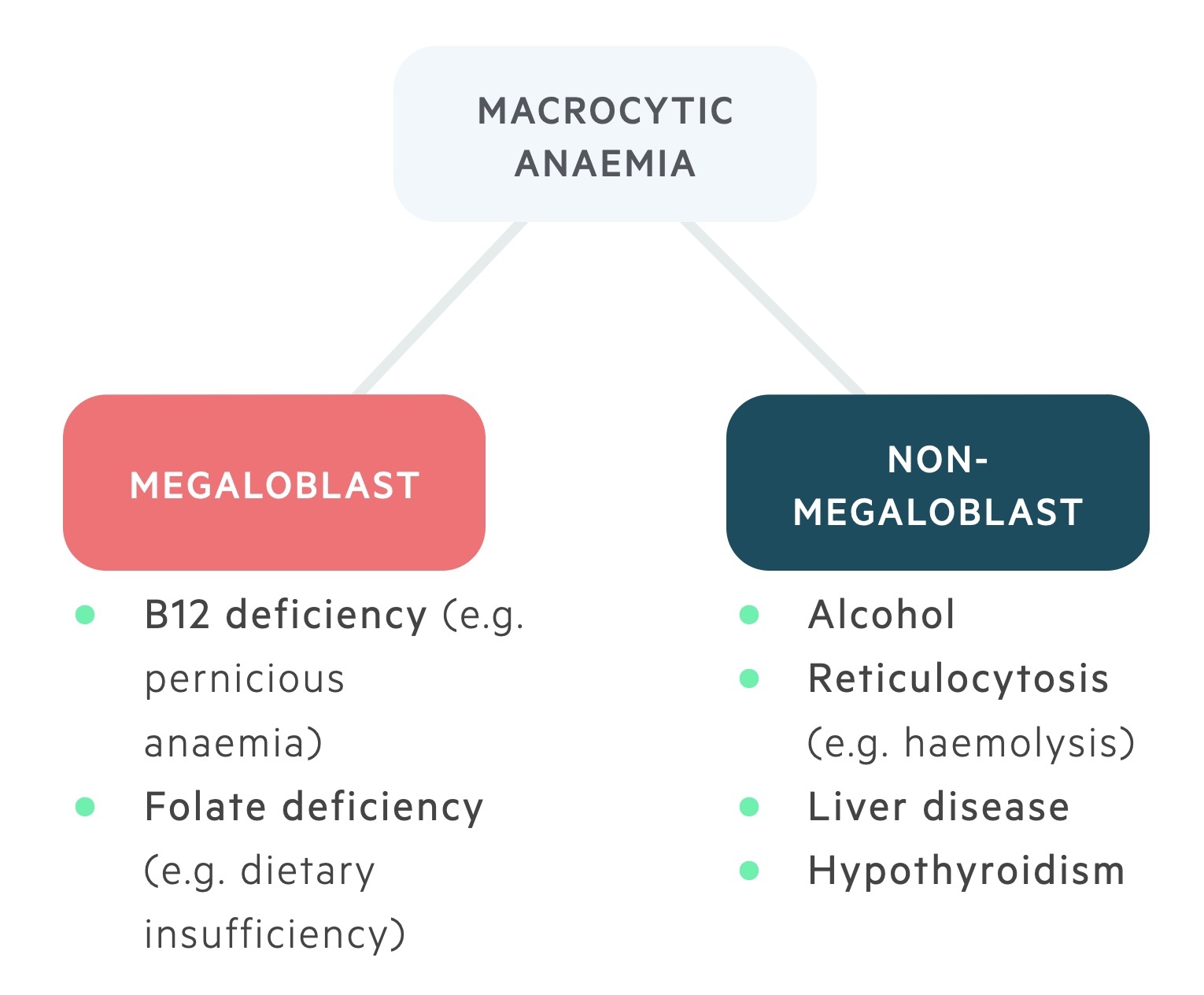
There are numerous causes of a macrocytic anaemia. These can be divided into the megaloblastic (which include B12 and folate deficiency) and non-megaloblastic anaemias (which include a wide range of pathologies).
Clinical features
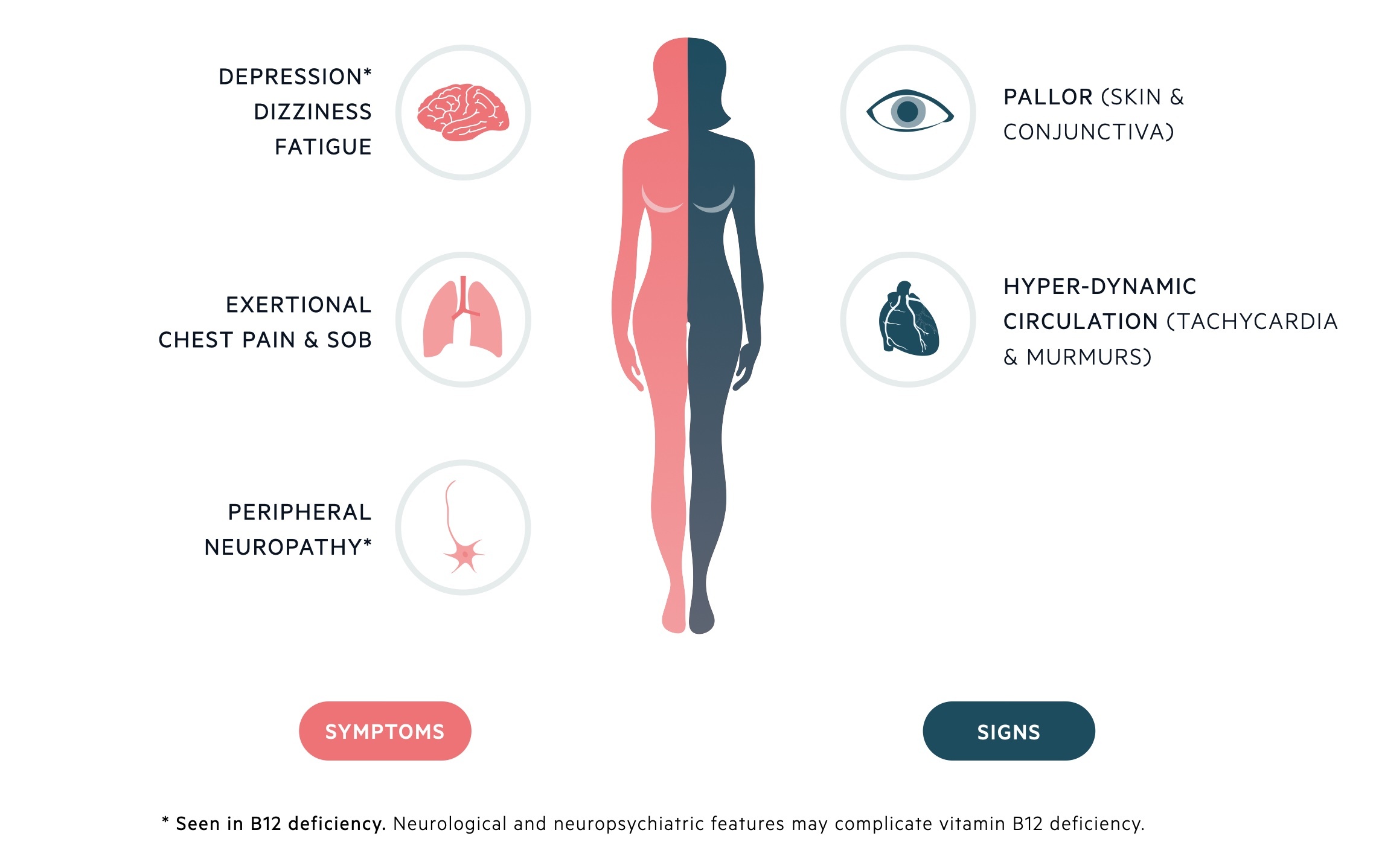
Clinical features are dependent upon the absolute degree of anaemia & rate of decline in Hb concentration.
Symptoms
- Fatigue
- Weakness
- Paraesthesia
- Dyspnoea
- Gastrointestinal symptoms (e.g. nausea, dyspepsia)
- Weight loss
Signs
- Atrophic glossitis
- Pallor
- Fever
- Splenomegaly
- Evidence of underlying disease
Megaloblastic anaemias
Megaloblasts are immature red cells with large nuclei seen in B12 & folate deficiency.
Megaloblastic anaemia is characterised by the development of megaloblastic erythropoiesis, in which abnormal erythrocyte progenitors are seen (megaloblasts).
Both folate and B12 are key to normal DNA synthesis. In deficiency states, abnormal DNA synthesis leads to impairment of cell maturation, resulting in immature cells with abnormally large nuclei.
Significant disturbance in cellular proliferation leads to degradation whilst still in the bone marrow. As a result, there is erythroid hyperplasia with ineffective erythropoiesis - leading to anaemia.
Other cell lines may also be affected. Another common feature is the generation of hypersegmented neutrophils. Typically, in megaloblastic erythropoiesis, more than 3% of neutrophils will have greater than 5 nuclear segments (normal being 3-5).
However, it is important to note that this is not a diagnostic feature and may be seen in other conditions, such as renal failure.
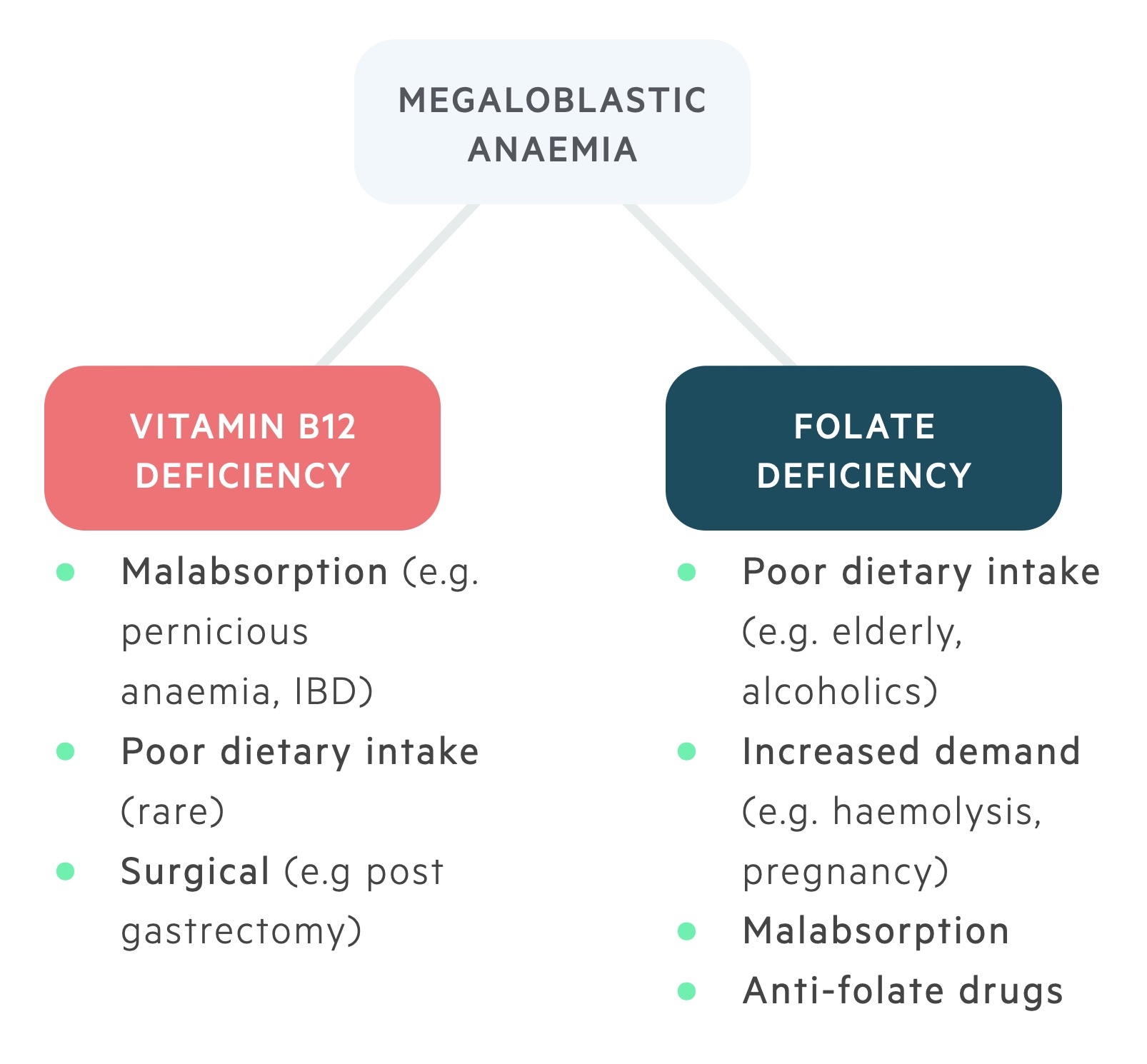
Vitamin B12 deficiency
Vitamin B12 (cobalamin) is found in meats and diary products. It is an essential vitamin for DNA synthesis in cells undergoing rapid proliferation.
Epidemiology
Vitamin B12 is predominantly found in meat and dairy products (due to bacterial synthesis) and is not present in plants. Thus, dietary deficiency is uncommon and typically seen only in strict vegans.
The overall burden of vitamin B12 deficiency increases with age but is less common than folate deficiency. Unlike folate, vitamin B12 stores last for years before deficiency develops.
Aetiology & pathophysiology
Absorption of vitamin B12 is a complex process; it is aided by a key glycoprotein, intrinsic factor (IF).
Parietal cells, found in the gastric epithelium, secrete IF. IF binds to vitamin B12 within the intestines. The subsequent vitamin B12-IF complex binds to receptors within the terminal ileum where it is absorbed.
Causes of vitamin B12 deficiency include:
- Inadequate intake (e.g. strict vegetarians, vegans)
- Inadequate secretion of intrinsic factor (e.g. pernicious anaemia, gastrectomy)
- Malabsorption (e.g. Crohn’s, tropical sprue)
- Inadequate release of B12 from food (e.g. gastritis, alcohol abuse)
Vitamin B12 deficiency is most commonly due to pernicious anaemia (PA).
Clinical features
Signs & symptoms may reflect the underlying cause & neuropsychiatric complications.
Neurological features
- Peripheral neuropathy
- Subacute degeneration of the cord
- Focal demyelination
Psychiatric features
- Depression
- Personality change
- Memory loss
Investigations
Blood film reveals a megaloblastic anaemia +/- hypersegmented neutrophils.
Investigations help demonstrate the reduction in vitamin B12 and the development of anaemia. These include full blood count (FBC), blood film, haematinics, lactate dehydrogenase (LDH) and liver function tests (LFTs).
Blood tests usually reveal profoundly macrocytic anaemia with a raised MCV. Bilirubin and LDH may be slightly elevated - indicating increased turnover of abnormal progenitors in the bone marrow. If a bone marrow aspirate is taken, megaloblastic erythropoiesis, marked erythroid hyperplasia with ineffective erythropoiesis with the development of giant metamyelocytes, may be demonstrated.
Other investigations are directed towards identifying the underlying cause of vitamin B12 deficiency. These can include: Schilling's test, gastroscopy and serological assessment (e.g. autoantibodies seen in PA).
Management
Management involves B12 replacement & treatment of the underlying cause.
Treatment typically takes the form of intramuscular hydroxocobalamin. A dose of 1 mg, three times a week for two weeks, followed by maintenance of 1 mg every three months.
Importantly, in patients with co-existing folate deficiency, B12 must be replaced first as folate replacement in this setting may precipitate neurological complications (e.g. subacute degeneration of the cord).
Subacute degeneration of the cord
Degeneration of the posterior & lateral columns of the spinal cord.
Traditionally, presents insidiously with numbness, weakness and paraesthesia affecting the lower extremities in a symmetrical manner. As the condition progresses, it can lead to ataxia (unsteady, uncoordinated movement), sensory deficit and autonomic dysfunction (e.g. bladder/bowel dysfunction). These features may be accompanied by peripheral nerve involvement.
On examination, dorsal column involvement is demonstrated by loss of vibrational sense, proprioception (position sense) and ataxia. Involvement of the lateral columns can lead to pyramidal weakness, hyperreflexia and upgoing planters (positive Babinski sign).
Rather confusingly, lateral column involvement, and hence pyramidal tract involvement, may lead to mixed neurological signs (e.g. extensor plantar response with reduced/absent ankle reflexes) due to coexisting peripheral neuropathy. Other features include visual disturbance, memory impairment and psychiatric symptoms.
Pernicious anaemia
Pernicious anaemia (PA) refers to vitamin B12 deficiency as a result of autoimmune destruction of the gastric epithelium.
Relatively common amongst Northern Europeans, with a high prevalence in those aged 60-70 years old.
Aetiology
Patients with PA typically develop chronic gastric inflammation, which may lead to gastric atrophy. Over time, the basal secretion of IF is severely decreased leading to the development of vitamin B12 deficiency.
Auto-antibodies
Associated with the development of a number of auto-antibodies:
- Anti-parietal cell antibodies - directed against the parietal cell membrane; sensitive, but not very specific.
- Anti-IF antibodies - directed against intrinsic factor; sensitivity less than that of anti-parietal cell antibodies (est. 50-70%), but a specificity of almost 100%.
Schilling’s test
Schilling’s test is the classic investigation for the diagnosis of PA - though is now rarely used in clinical practice. The test is divided into two stages:
- Stage 1: Ingestion of oral radiolabelled vitamin B12 followed by an IM injection of unlabelled vitamin B12. The IM injection is given to prevent radiolabelled B12 binding in vitamin B12 depleted tissue. A 24-hour urine collection is then taken, which should show an 8-40% excretion of radiolabelled vitamin B12 in normal subjects. A low level of radiolabelled vitamin B12 excretion indicates abnormal absorption.
- Stage 2: If stage 1 is abnormal, the test can be repeated 3-7 days later. The only addition is an oral administration of IF at the same time. If there is an increase in the excretion of radiolabelled vitamin B12 the cause is secondary to IF deficiency (i.e. PA). If there is no increase in the excretion of radiolabelled vitamin B12 the cause is secondary to malabsorption at the terminal ileum.
Malignancy risk
Patients with pernicious anaemia have an increased risk of gastric malignancy. The role of routine screening is currently inconclusive, but patients do warrant an initial assessment of the upper GI tract with endoscopy at the time of diagnosis.
Folate deficiency
Folate (vitamin B9) is an important molecule which acts as a cofactor in amino acid metabolism and DNA/RNA synthesis.
Folate is commonly found in a variety of food sources, but due to its rate of loss being 10-20 times greater than B12, deficiency states can develop much more rapidly.
Epidemiology
In general, megaloblastic anaemia and folate deficiency are seen most commonly in countries where malnutrition is problematic.
Exact epidemiology of folate deficiency is not well known, but high-risk patient groups include: children, pregnant women and the elderly.
Aetiology & pathophysiology
Folate is found in both animals and plants, and the daily requirement of folate is approximately 100-200 micrograms per day.
Absorption of folate occurs within the proximal part of the small intestines (e.g. duodenum & jejunum).
There are plenty of hepatic stores of folate (approx. 8-20 mg), but this reserve is lost rapidly from cellular metabolism and the shedding of epithelial cells. There is an estimated loss of 1-2% of stores per day. Therefore, folate deficiency can develop after months, compared to vitamin B12 deficiency, which tends to develop over years.
Causes of folate deficiency:
- Inadequate dietary intake
- Malabsorption (e.g. coeliac disease, Crohn's disease)
- Increased requirements (e.g. pregnancy, malignancy)
- Increased loss (e.g. Chronic liver disease)
- Other (e.g. anti-convulsants, ETOH abuse)
Inadequate dietary intake of folate is extremely common, especially seen in elderly patients. As folate absorption occurs in the upper part of the small intestines, conditions that affect this area of the bowel (e.g. Coeliac disease) commonly lead to deficiency.
Clinical features
Features of folate deficiency are primarily related to the underlying anaemia. Clinical features may include weakness, fatigue, pallor and other evidence of malnutrition.
Other clinical features will be related to the underlying cause of folate deficiency. For example, in Coeliac disease, there may be evidence of gastrointestinal symptoms such as diarrhoea, bloating, dyspepsia and abdominal discomfort.
Investigation
Diagnosis of folate deficiency is based on a series of blood tests, which show the development of megaloblastic anaemia with a high MCV and low folate stores.
Blood tests include: FBC, blood film, haematinics and red cell folate. Red cell folate is a better measure of levels than serum folate, since levels are affected even with a short period of deficiency.
Importantly, levels of folate may be affected by vitamin B12 deficiency and it may be difficult to distinguish between the two.
In general, normal levels of vitamin B12 make the diagnosis of folate deficiency most likely. If levels of vitamin B12 are normal/reduced then a Schilling's test can help differentiate. In clinical practice, a trial with folic acid is often employed.
If B12 deficiency cannot be completely ruled out, then replacement of B12 and folate should occur together. This is because replacement of folic acid only in the presence of vitamin B12 deficiency may cause significant neurological disease.
Management
General management of folate deficiency is to treat the underlying cause if required and to provide supplemental folic acid.
Folic acid is usually given as a once daily oral dose of 5 mg for up to four months. Levels can be repeated in primary care. Importantly, pregnant women should receive folate supplementation prior to conception until 12 weeks (400 mcg or 5 mg daily) due to the risk of neural tube defects.
Non-megaloblastic anaemias
A group of macrocytic anaemias that do not lead to megaloblastic erythropoiesis.
Causes of non-megaloblastic anaemia are varied and include:
- Chronic alcohol use
- Hypothyroidism
- Haemolytic anaemia
- Medications (e.g. cyclophosphamide)
- Haematological malignancies
Chronic alcohol use is the most common cause of a non-megaloblastic anaemia. It is thought to be due to the toxic effects of acetaldehyde on erythrocyte progenitors.
Haemolytic anaemia may lead to macrocytic anaemia due to the increased release of reticulocytes from the bone marrow. Reticulocytes are immature RBCs that are bigger in diameter and contain remnant nuclear material.
Many medications may be implicated in the development of macrocytic anaemia, usually from the inhibition of metabolic pathways needed for erythropoiesis. Causative drugs include cyclophosphamide, hydroxyurea and azathioprine.
Last updated: April 2021
Have comments about these notes? Leave us feedback
