Hepatitis C
Notes
Overview
Hepatitis C is caused by the hepatitis C virus (HCV), which is a major cause of chronic liver disease worldwide.
Traditionally, hepatitis C virus (HCV) infection occurred followed blood transfusions or in association with intravenous drug use. It was an unknown pathogen causing a non-A, non-B hepatitis leading to chronic liver disease and hepatocellular carcinoma (HCC). This is now known as hepatitis C, which is caused by the small RNA virus hepatitis C.
HCV leads to chronic infection in 75-80% of individuals and accounts for significant morbidity and mortality from cirrhosis and HCC. HCV is now a curable condition with a range of direct-acting antivirals (DAA) that can be used against the virus.
Acute versus chronic
Hepatitis C may cause acute or chronic infection:
- Acute: presence of hepatitis C following incubation period (time from virus acquisition to clinical symptoms). Majority are asymptomatic (> 60%) and 15-45% will clear the virus spontaneously.
- Chronic: failure to clear the virus after acute infection. Defined as presence of HCV for > 6 months. Around 10-20% will develop complications including cirrhosis and HCC over a 20-30 year period.
Hepatitis C virus
Hepatitis C virus is a small, enveloped RNA virus that has eight genotypes.
HCV belongs to the family of Flaviviridae viruses that also includes Yellow fever, West Nile and Dengue virus. It is a RNA virus that contains 9400 nucleotides with one long open-reading frame (part translated into a protein). The enveloped particle is approximately 56-65 nm in diameter.
Genome
HCV is a positive-sense RNA virus that contains 9400 nucleotides. Its one open-reading frame encodes a long polypeptide that is cleaved into several smaller polypeptides. It comprises eight genotypes (1a-c, 2, 3, 4, 5, 6, 7, 8) and hundreds of subtypes. Genotypes 5, 7, and 8 account for < 1% of global cases. The predominant genotype differs by geographical location.
Proteins
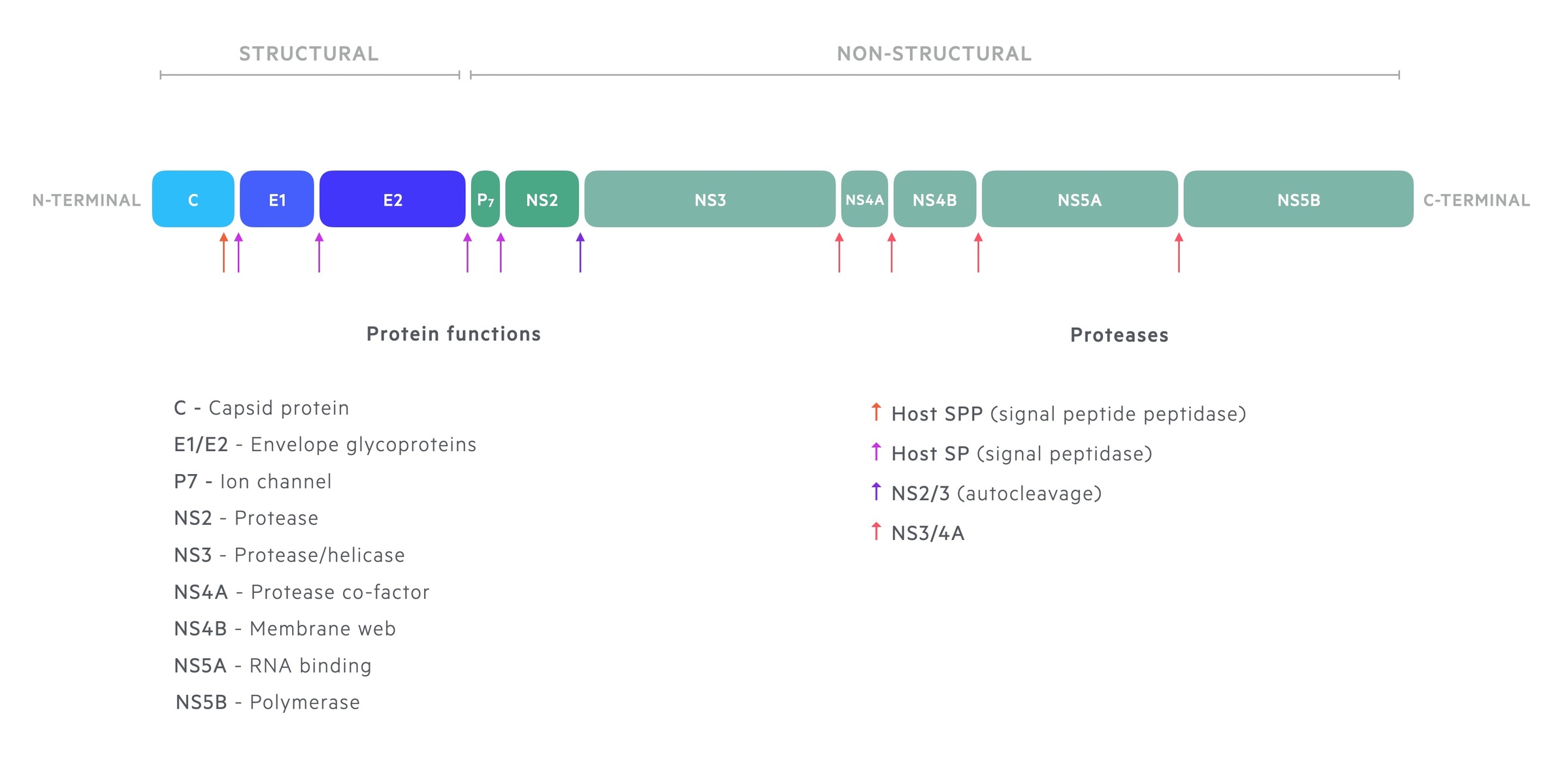
The single translated polyprotein is cleaved by cellular and viral proteases into ten proteins. These are divided into structural and non-structural proteins.
- Structural proteins: Core, E1, E2
- Non-structural proteins: p7, NS2, NS3, NS4A, NS4B, NS5A, NS5B
The core protein links with genomic RNA to form the nucleocapsid for viral particles. The structural proteins E1 and E2 are found in the viral envelope. The non-structural proteins are predominantly involved in RNA replication and regulation of cell function.
- NS2: protease activity. Autocleavage at the NS2/3 site
- NS3: ATPase/helicase activity (involved in RNA replication) and protease activity.
- NS4A: co-factor in NS3 activity
- NS4B: helps induce a “membranous web”. Locates viral proteins for replication.
- NS5A: contribution to viral replication
- NS5B: viral RNA-dependent RNA polymerase
Replication
The whole replication cycle is complex and not completely understood.
Importantly, HCV replicates using an error-prone RNA-dependent RNA polymerase (NS5B). Due to the rapid turnover and lack of ‘proof-reading’ the population of viruses generated by replication are closely related but with different nucleotides sequences. This difference in the population is referred to as a ‘quasispecies’. This contributes to the viruses ability to evade the immune system.
Epidemiology
Worldwide, hepatitis C is a major health problem.
Globally, it is estimated that > 185 million people are infected with HCV. In the UK, 143,000 individuals were living with hepatitis C in 2018. The current burden of hepatitis C stems from millions of patients being infected before discovery of the virus in 1989 and implementation of universal precautions including screening blood donors.
There are significant geographical differences in the prevalence of hepatitis C. Six countries accounts for >50% of all infections that include China, Pakistan, Nigeria, Egypt, India, and Russia. New infections are largely driven by intravenous drug users (IVDU) also referred to as people who inject drugs (PWID). There is a major problem with co-infection with human immunodeficiency virus (HIV) and hepatitis B virus (HBV).
Transmission
HCV can be transmitted by direct percutaneous exposure to blood (e.g. blood transfusion, injecting drugs).
There are three main routes of transmission of HCV:
- Parenteral: transmission occurring outside the alimentary (GI) tract. Usually through percutaneous injection.
- Permucosal: transmission via a mucous membrane. Typically during sexual contact.
- Vertical: mother to the infant during pregnancy, at the time of delivery, or during the first 28 days after birth.
Parenteral
In the UK and western world, IVDU/PWID account for the majority of parenteral transmission. This includes both past and current users because of the delay between acquisition and development of features of chronic liver disease.
Traditionally, infected blood products were a major cause of transmission. However, in the UK (like many countries) there is now universal blood donor screening for HCV among other viruses that was introduced in the early 1990's. When determining risk of hepatitis C, it is important to ask about the year of any blood transfusions and countries of administration.
Other parenteral routes of transmission include:
- Health care workers: needle stick injuries
- Parenteral exposure to blood
- Unsafe medical practice (e.g. unsafe injections, contaminated instruments)
- Tattooing and ear piercing
- Acupuncture
Blood donor screening
Due to the rise in cases of human-immunodeficiency virus (HIV) in the 1980s, blood donors were screened for HIV from 1986. Five years later, with the rising epidemic of HCV, blood was also screened for HCV. Blood donors are now screened for a variety of infections including:
- Syphilis
- HIV
- Hepatitis B virus
- Hepatitis C virus
- Hepatitis E virus
- Human T-lymphotropic virus (HTLV)
- Others: selected testing may be completed depending on travel history
Natural history
The majority of patients who acquire HCV will develop chronic hepatitis.
After exposure to HCV the incubation period (time between exposure and first development of symptoms) is usually 2-12 weeks. Around 15-40% of patients will spontaneously clear the virus, which normally occurs within four months. During this acute phase, many patients will be asymptomatic or ‘subclinical’. This means most patients fail to clear the virus and will go onto develop chronic hepatitis, which can lead to cirrhosis and HCC.
The normal host response to viruses, such as HCV, is expression of interferons (e.g. IFN-alpha). IFN-alpha is induced by double-stranded RNA and binds to interferon receptors. This leads to down stream responses including inhibition of protein synthesis, inactivation of viral RNA, and enhancement of phagocytic and cytotoxic mechanisms. HCV is able to evade these normal responses through a variety of mechanisms helping it establish chronic infection. The natural target of HCV are hepatocytes.
Once chronicity of hepatitis C is established there seems to be a weaker immune response. This may in part be due to the large number of mutations that occur from the poor proof-reading by viral polymerase during replication. This enables the virus to escape host detection by T lymphocytes. There are two hypervariable regions on its structural E2 protein that has an extremely high rate of spontaneous mutation.
Over time, there is activation of hepatic stellate cells by the inflammatory process leading to fibrosis. Factors that are associated with an accelerated progression to advanced fibrosis and cirrhosis include older age, male gender, heavy alcohol intake and co-infection (Hepatitis B, HIV). Patients with cirrhosis are at increased risk of HCC and decompensated liver disease. Approximately 15-20% of patients with liver disease will die within the first year of decompensation.
Clinical features
In chronic hepatitis C, the majority of patients are asymptomatic.
The clinical features of hepatitis C depend on the stage of illness.
- Acute hepatitis: usually mild or asymptomatic.
- Chronic hepatitis: majority asymptomatic, picked up on screening.
- Decompensated cirrhosis: characterised by jaundice, ascites, confusion, GI bleeding and coagulopathy.
Acute hepatitis C
Acute hepatitis C is usually mild and often ‘subclinical’. This means it goes unnoticed by the patient. Fulminant hepatitis (i.e. acute liver failure) is rare in acute hepatitis C.
Features of acute hepatitis C:
- Fatigue
- Lethargy
- Anorexia
- Right upper quadrant pain
- Jaundice (25%): more likely to clear the virus if present
Chronic hepatitis C
Progression from acute to chronic hepatitis C will usually go unnoticed. The condition slowly develops over many years with cirrhosis developing within 20 years in 10-20% of patients.
The majority of patients with chronic hepatitis C are asymptomatic. Patients may have non-specific symptoms including fatigue or malaise. Other patients may have features of chronic liver disease that are collectively referred to as the ‘stigmata of chronic liver disease’. For more information see Chronic liver disease notes.
Decompensated cirrhosis
Decompensated cirrhosis refers to an inability of the liver to carry out normal function. It is characterised by development of ascites, encephalopathy, jaundice, coagulopathy and GI bleeding.
For more information see Chronic liver disease notes.
Extra-hepatic manifestations
This refers to a variety of clinical presentations occurring outside the liver that are typically seen in chronic hepatitis C:
- Mixed cryoglobulinaemia: systemic inflammation due to the presence of cryoglobulins in the serum. These are immunoglobulins that precipitant at low temperatures. Associated with digital ulcers, Raynaud’s, purpura and peripheral neuropathy. It is considered a type of lymphoproliferative disorder.
- Porphyria cutanea tarda (PCT): one of the porphyrias. These refer to conditions characterised by excess porphyrins that are needed for heme biosynthesis. PCT is due to decreased activity of hepatic uroporphyrinogen decarboxylase. Characterised by cutaneous photosensitivity, hirsutism and scleroderma-like changes (i.e. hardening of skin).
- Glomerulonephritis: due to immune complex deposition within glomeruli. Commonly associated with membranoproliferative glomerulonephritis. Less commonly membranous glomerulonephritis. For more information see Glomerulopathies notes.
- Non-Hodgkin’s lymphoma (NHL): increased risk of NHL, particularly in untreated patients. Associated with B-cell subtypes of NHL (e.g. diffuse large B cell lymphoma). For more information see Non-Hodgkin’s lymphoma notes.
- Others (e.g. lichen planus, sialoadenitis, Sicca syndrome).
Diagnosis
The diagnosis of both acute and chronic hepatitis C is based on anti-HCV antibodies and serum HCV RNA levels.
The diagnosis of hepatitis C infection can utilise both detection of HCV RNA levels and antibodies directed against HCV (anti-HCV antibodies). Anti-HCV antibodies form the first-line test. If positive, patients should be tested for HCV RNA levels. This should take into account timing (anti-HCV antibodies are unlikely to be present in early infection) and co-morbidities (patients with profound immunosuppression may not form antibodies).
Acute hepatitis C
In acute hepatitis C, the incubation period is 2-12 weeks and the HCV RNA levels usually become positive within 2 weeks of exposure. During this early period seroconversion (formation of antibodies) may not have occurred so anti-HCV antibodies may be negative. A confident diagnosis can only be made with documentation of recent seroconversion.
If anti-HCV antibodies are negative, HCV RNA levels may be used to aid the diagnosis. However, their presence varies widely in the acute phase. This means that if the clinical picture is in keeping with hepatitis but initial tests are negative, HCV RNA levels should be repeated at 12 and 24 weeks.
Chronic hepatitis C
In chronic hepatitis C, the presence of both elevated HCV RNA levels and positive anti-HCV antibodies is consistent with the diagnosis. In suspected new acquisition of hepatitis C, the presence of elevated HCV RNA levels beyond 6 months is consistent with chronic infection because spontaneous clearance beyond this point is rare.
Screening
Screening for hepatitis C using anti-HCV antibodies is important because many patients remain undiagnosed as the condition is largely asymptomatic until complications develop (e.g. decompensated cirrhosis, HCC).
Different regions may have different screening recommendations (e.g. universal testing on all accident and emergency attendances or screening at risk patients such as intravenous drug users).
Genotyping
There are six genotypes of hepatitis C. Identification of the genotype and subtype should be completed prior to initiation of treatment. This is because knowledge of the genotype alters treatment choice. There are options to use pangenotype regimens if genotyping is not available.
Investigations
A full work-up is required to determine the degree of liver damage, assess co-morbidities and guide treatment.
Treatment options for hepatitis C depend on co-existing liver diseases, co-morbidities and degree of liver injury (e.g. presence or absence of cirrhosis). Therefore, a full work-up is required for patients with suspected or confirmed hepatitis C.
Remember, it is important to determine alcohol consumption and drug use as part of the assessment.
Routine investigations
- Full blood count: anaemia and thrombocytopaenia may reflect underlying cirrhosis
- Urea & electrolytes: coexistent renal impairment
- Liver function tests: degree of necroinflammation
- Clotting profile: coagulopathy associated with poor synthetic function
Non-invasive liver screen
A non-invasive liver screen refers to screening questions, biochemical tests and imaging to assess patients with suspected liver disease. This should be completed in all patients with suspected or confirmed hepatitis C. The full list of appropriate tests can be found in Chronic liver disease notes.
Liver ultrasound is a useful non-invasive imaging modality to look for features of cirrhosis and liver lesions (e.g. HCC).
Non-invasive fibrosis testing
The degree of fibrosis is important to quantify because it impacts on treatments that can be offered to patients. There are a number of non-invasive methods that can be used, which negates the need for invasive liver biopsy. These are used to predict the presence of advanced fibrosis or cirrhosis, which is graded according to the METAVIR score (F0-F4).
Transient elastography refers to a quick, painless test that assesses liver stiffness. It measures sound wave velocity using an ultrasound probe to indicate the ‘degree’ of fibrosis and thus likelihood of cirrhosis. The elastography score in kPa equates to a grade of fibrosis.
Invasive tests of fibrosis
Liver biopsy (percutaneous or transjugular) can be completed to assess the extent of inflammation and fibrosis within the liver. A liver biopsy is usually not required, but may be performed if a mixed pathology is suspected (i.e. liver disease due to a combination of pathologies).
Management overview
Over the last decade, treatment of hepatitis C has changed dramatically and the goal is now to cure HCV infection.
With the introduction of many new direct-acting antivirals (DAA) therapies, HCV is a curable disease.
Indications
Treatment should be offered to all patients, unless contra-indications, in order to:
- Prevent development of advanced fibrosis, cirrhosis, severe extrahepatic manifestations and death
- Prevent and reduce the risk of HCC
- Reduce the rate of hepatic decompensation
- Prevent onward transmission of HCV
- Improve quality of life and remove stigma
This includes patients who were previously treated with interferon- or ribaviron-based therapies (often referred to as ‘treatment-experienced’ patients).
Sustained virological response
The goal of using DAAs is to achieve a sustained virological response (SVR), which is monitored using HCV RNA levels. Levels should be measured at baseline, 12 weeks and 24 weeks following therapy. SVR is defined as absent viraemia at 24 weeks post treatment for chronic hepatitis C.
Direct-acting antivirals
Numerous anti-viral drugs are available, often in combination, to treat acute and chronic hepatitis C.
Direct-acting antivirals (DAA) primarily target HCV-encoded proteins that are important in viral replication. These include NS3/4A inhibitors, NS5A inhibitors and NS5B polymerase inhibitors. Different drug combinations can be used as part of a pangenotype regimen (i.e. treating blindly) or genotype-specific regimen (i.e. targeting the specific genotype).
NS3/4A inhibitors
NS3/4A is a serine protease involved in viral replication. Inhibitors block the catalytic site on NS3. In Europe, the following inhibitors are available:
- Glecaprevir: given in combination with Pibrentasvir (NS5A inhibitor)
- Grazoprevir: given in combination with Elbasvir (NS5A inhibitor)
NS5A inhibitors
NS5A is involved in both viral replication and assembly. However, the precise mechanisms are still unknown and thus the exact mechanism of the inhibitors is also unknown. In Europe, the following inhibitors are available:
- Elbasvir: given in combination with grazoprevir (NS3/4A inhibitor)
- Velpatasvir: available in combination with Sofosbuvir (NS5B inhibitor)
- Pibrentasvir: given in combination with Glecaprevir (NS3/4A inhibitor)
NS5B polymerase inhibitors
NS5B is a RNA-dependent RNA polymerase that is critical for viral replication. It is a highly conserved protein across all genotypes of hepatitis C. There are two types of inhibitors to this protein:
- Nucleot(s)ide inhibitors: compete with nucleotides for polymerase binding and result in chain termination
- Non-nucleoside inhibitors: targets allosteric sites away from the active site that alter its conformation and prevent binding.
The following inhibitors are example options:
- Sofosbuvir: nucleot(s)ide inhibitor. Various combinations available.
- Dasabuvir: non-nucleoside inhibitor.
Acute hepatitis C management
Patients with acute hepatitis C should be considered for treatment to prevent risk of chronic infection.
Patients should be offered a combination of DAAs based on genotype for 8 weeks. HCV RNA levels should be checked at 12 and 24 weeks following treatment to monitor for a SVR.
Post-exposure prophylaxis is not indicated in hepatitis C unless there is documented HCV transmission.
Chronic hepatitis C management
Treatment of chronic hepatitis C depends on the genotype and degree of liver disease.
When offering treatment for chronic hepatitis C, consideration should be made to the genotype, degree of liver disease and previous treatment regimens. Adjustments may also be required due to drug interactions and co-morbidities.
Treatment of hepatitis C, like hepatitis B, is specialist and should involve a viral hepatologist with access to a multi-disciplinary team. The choice of treatment is complex and well beyond the scope of these notes. Extensive guidelines by the European Association for the Study of the Liver (EASL) are available for further reference.
Here, we summarise some of the key points regarding treatment.
Combination therapy
A variety of combination regimens are available to treat patients with chronic hepatitis C. These are generally given for 8 or 12 weeks depending on the choice of agents. The goal is to achieve sustained virological response at 12 or 24 weeks following treatment.
Options include:
- Pangenotypic drug combinations
- Sofosbuvir/velpatasvir (SOF/VEL)
- Glecaprevir/pibrentasvir (GLE/PIB)
- Sofosbuvir/velpatasvir/voxilaprevir (SOF/VEL/VOX)
- Genotype-specific drug combinations
- Sofosbuvir/ledipasvir (SOF/LDV)
- Grazoprevir/elbasvir (GZR/EBR)
Genotype
Treatment regimens can be based on an identified genotype or given as a ‘blind’ pangenotypic regimen that has good efficacy against most, if not all, genotypes. The use of pangenotypic regimens is particularly important to improve access to treatment worldwide by reducing costs and simplifying treatment choices.
Two commonly prescribed pangenotypic regimens are Sofosbuvir/velpatasvir and Glecaprevir/pibrentasvir. These are both given as 12 weeks regimens in patients with or without compensated cirrhosis (Child-Pugh A). This achieves a SVR in 95% of treated individuals. The only tests that are needed to initiate treatment with a pangenotypic regimen is a positive HCV RNA (or core antigen testing if viral RNA is not available) and calculation of a non-invasive marker of fibrosis (e.g. FIB-4). This latter component is particularly important to identify patients who need ongoing HCC surveillance post treatment.
Degree of liver disease
It is important to assess for the degree of fibrosis in patients before initiation of treatment. This usually involves non-invasive imaging with transient elastography. It enables identification of patients who will need HCC surveillance following treatment and those with cirrhosis who made need alteration to treatment.
Patients with compensated cirrhosis (Child-Pugh A) can generally have the normal recommended regimens. In patients with decompensated cirrhosis (Child-Pugh B or C), protease inhibitor-containing regimens are contraindicated because they can worsen decompensation. It is also important to consider suitability of patients with decompensated cirrhosis for transplantation, if appropriate, according to national transplantation guidelines. If accepted, the timing of treatment may change depending on the estimated date of transplant.
Specific groups
Treatment of hepatitis C may need to take into account specific groups when considering treatment options. These groups include:
- Patients accepted for liver transplantation
- Co-infection with Hepatitis B virus
- Co-infection with HIV
- Severe extrahepatic manifestations
- Severe renal impairment or end-stage renal disease
Follow-up
A SVR is associated with overall improvement in the degree of inflammation and fibrosis in patients without cirrhosis. Patients with advanced fibrosis or cirrhosis are still at risk of life-threatening complications (e.g. HCC). These patients need to remain under surveillance for HCC with 6 monthly liver ultrasounds +/- alpha-fetoprotein (AFP).
Patients who achieve a SVR and have only moderate fibrosis at worst can be considered for discharge without need for specialist follow-up. It is important to consider other co-morbidities that may require periodic follow-up (eg. NAFLD, alcohol excess), or following up patients who still engage in high risk behaviour due to risk of reinfection.
Prognosis
In patients with chronic hepatitis C, cirrhosis develops in up to 20% within 20 years of disease onset.
The major risks associated with chronic hepatitis C include cirrhosis, decompensated cirrhosis and HCC. Every year, 1-3% of patients with cirrhosis will develop a HCC.
Factors associated with progression to cirrhosis and HCC include excess alcohol intake, hepatitis B co-infection, HIV co-infection and other causes of immunosuppression.
Last updated: July 2021
Have comments about these notes? Leave us feedback
