Gonorrhoea
Notes
Overview
Gonorrhoea is a common sexually transmitted infection caused by the bacterium Neisseria gonorrhoeae.
Gonorrhoea is a common sexually transmitted infection (STI) that is transmitted by direct contact with infected secretions. It is caused by the gram negative organism Neisseria gonorrhoeae.
Gonorrhoea causes infection of mucous membranes, most notably the urethra, endocervix, rectum, pharynx and conjunctiva. Rarely, it can cause disseminated infection.
Epidemiology
Gonorrhoea is most commonly seen among patients 15-24 years old.
Rates of gonorrhoea have been increasing with 70,936 cases in the UK in 2019, which is a 26% increase from 2018. This accounts for around 15% of all new cases of STIs. Chlamydia accounts for almost 50% of cases.
Gonorrhoea is most commonly seen among men who have sex with men (MSM).
Aetiology & pathophysiology
Gonorrhoea is caused by the gram negative diplococcus Neisseria gonorrhoeae.
The causative organism of gonorrhoea is the gram negative, intracellular, diplococcus N. gonorrhoeae. The primary site of infection are mucous membranes that are lined with columnar epithelium including the urethra, endocervix, rectum and pharynx. It is spread by direct contact with infected secretions. Infections of the conjunctiva are usually due to self-inoculation.
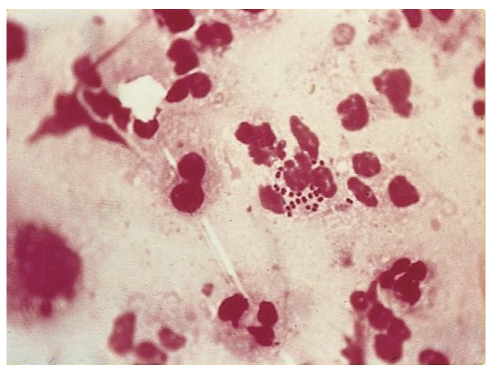
Gram stain showing the diplococci of N. gonorrhoeae
Image courtesy of SOA-AIDS Amsterdam
Lower tract infections
The bacteria are able to attach to host cells via pili (hair-like appendages) and penetrate between epithelial cells. Here, it establishes infection in the lower urogenital tract causing urethritis in males and endocervicitis in females. Infection of the pharynx and rectum are more likely to be asymptomatic.
Upper tract infections
The bacteria can spread retrograde to cause an upper urogenital infection leading to pelvic inflammatory disease (PID) in women and epididimyo-orchitis or prostatitis in men. PID may occur in up to 20% of women with endocervicitis and can be associated with endometritis, salpingitis and tubo-ovarian abscess.
Disseminated infection
It is estimated that 1% of gonococcal infections result with haematogenous dissemination. Both host and bacterial factors influence the likelihood of disseminated infection. Disseminated infection is associated with systemic features including fever, arthralgia, myalgia, migratory polyarthritis and rash.
Sites of dissemination can include:
- Joints: septic arthritis
- Tendons: tendinitis and tenosynovitis
- Brain: meningitis
- Heart: endocarditis
Long-term complications
Untreated, gonorrhoea can cause long-term complications including tubal infertility, ectopic pregnancy and chronic pelvic pain. These are usually in association with PID.
Clinical features
Penile discharge is observed in > 90% of men with gonorrhoea but only 50% of women are symptomatic.
Urogenital infections
In men, >90% are symptomatic and most commonly present with penile discharge. This usually occurs 2-5 days following infection.
Women most commonly present with endocervicitis that is characterised by cervical inflammation and mucopurulent discharge. Only 50% of women are symptomatic.
- Symptoms
- Women or transmen: Vaginal discharge, dysuria, lower abdominal pain (25%), abnormal bleeding (rare)
- Men or transwomen: mucopurulent urethral discharge, dysuria, testicular pain or swelling (rarely)
- Signs
- Women or transmen: cervicitis with mucopurulent discharge +/- endocervical bleeding (on speculum examination). Pelvic or abdominal pain is uncommon unless co-infection with chlamydia.
- Men or transwomen: Urethral discharge, testicular pain/swelling.
Rectal infections
Most cases of rectal gonorrhoea are asymptomatic. Symptoms can include:
- Anal pain
- Anal discharge
- Pruritus
Epididymo-orchitis
For more information see our notes on epididymo-orchitis.
Prostatitis
For more information see our notes on prostatitis.
Pelvic inflammatory disease
PID can complicate up to 14-20% of gonorrhoea cases.
Features include:
- Systemic upset: Fever, malaise, anorexia
- Symptoms: low abdominal pain, abnormal vaginal bleeding, vaginal discharge or cervical discharge
- Signs: marked abdominal pain, cervical excitation, mucopurulent discharge
- Long-term complications: infertility, ectopic pregnancy, chronic pelvic pain
- Short-term complications: Tubo-ovarian abscess, Fitz-Hugh-Curtis syndrome (see below)
Fitz-Hugh-Curtis syndrome
This is a rare complication of PID in some patients that presents with right upper quadrant pain due to peri-hepatitis. More common with chlamydia, but also seen with gonorrhoeal PID. Analgesia, antibiotics and treating the underlying PID is key.
Disseminated gonorrhoea
A small proportion of gonorrhoeal infections can lead to dissemination affecting multiple sites.
- Tendinitis (inflammation of tendon): pain, swelling, reduced range of movement
- Tenosynovitis (inflammation around tendon sheath): swelling, pain, reduced range of movement
- Septic arthritis: fever, joint pain, swelling, immobility
- Meningitis: fever, neck stiffness, headache, photophobia
- Endocarditis: fever, shortness of breath, chest pain, septic/immunological emboli
Diagnosis & investigations
Nucleic acid amplification tests (NAATs) are the principle diagnostic tests for gonorrhoea.
Diagnosis of gonorrhoea can be based on microscopy, culture or nucleic amplification tests (NAATs).
Microscopy
Microscopy following gram stain can be completed on samples taken from suspected lesions (e.g. urethra or cervix). Microscopy will show the typical gram negative diplococci of N. gonorrhoeae within polymorphonuclear leucocytes (see aetiology & pathophysiology image). Recommended for symptomatic males and those with rectal symptoms.
Typical samples:
- Penile meatal or urethral swabs: up to 95% sensitivity in symptomatic patients with urethral discharge.
- Female urethral swabs: 37-50% sensitivity compared to culture
- Endocervical swabs: 20% sensitivity compared to culture and only 16% sensitivity compared to NAATs
- Anorectal swabs: offered if rectal symptoms present.
Culture
Culture should be completed alongside swabs for microscopy to assess antibiotic susceptibility. This is important with the rising cases of antibiotic resistance. If diagnosis is based on NAAT, swabs should be taken for culture prior to treatment.
NAAT
NAATs have a high sensitivity for both asymptomatic and symptomatic gonorrhoeal infections (>95%). They detect infections by amplification of N. gonorrhoeae genetic material obtained from relevant samples.
- First-pass urine: equivalent sensitivity to urethral swabs, preferred method in men.
- Urethral swab: typically used for microscopy in men to give a rapid diagnosis. Can subsequently be sent for NAAT testing. Urine test preferred.
- Vulvo-vaginal swabs (VVS): preferred method in women. Better sensitivity compared to both endocervical swabs and urine samples.
- Endocervical swabs: requires speculum examination and swab of endocervix. More invasive and performs less well compared to VVS.
- Rectal swabs: should be completed routinely in MSM and anyone with rectal symptoms.
If the initial test is negative, but there has been significant sexual exposure in the last two weeks, patients will need a repeat test in two weeks if the decision is made not for empirical treatment.
Other investigations
Patients with suspected gonorrhoea require referral and assessment at a genitourinary medicine (GUM) clinic. This enables a full sexual health screen including testing for other STIs such as chlamydia, syphilis and HIV.
An estimated 19% of patients with gonorrhoea are co-infected with chlamydia.
Management
A single dose of parenteral ceftriaxone is the treatment of choice.
General advice
Patients should be advised to avoid sexual contact until both themselves and partners have completed treatment. This should be for a minimum of 7 days after completion of treatment.
Contract tracing
Contact tracing should be completed with advice from a GUM clinic. Among symptomatic male patients with urethral discharge, all partners within two weeks of symptoms should be notified. Among patients who are asymptomatic or have symptoms localised to other sites (e.g. rectum. endocervix, pharynx), all partners within three months of symptoms should be notified.
All notified partners should be encouraged to undertake a full sexual health screen. Treatment is initiated only if they test positive. Those presenting within two weeks of exposure are considered for empirical treatment depending on risk, otherwise repeat testing is offered if negative testing.
Test of cure
This refers to repeat testing after the course of treatment. Routine test of cure (TOC) is required for all patients including reassessment. This helps detect treatment failure, resistant cases, poor compliance, ensures symptom resolution and determine risk of reinfection. Choice of testing and timing is guided by the GUM clinic.
Pharmacological management
There has been significant changes in the treatment of gonorrhoea due to the rise in resistant strains. Choice of therapy depends on knowledge of antimicrobial susceptibility, site of infection and any complications.
Uncomplicated anogenital infection
- Ceftriaxone 1 g intramuscularly (IM) as a single dose (unknown antibiotic susceptibility)
- Ciprofloxacin 500 mg orally as a single dose (known antibiotic susceptibility)
A single dose of ceftriaxone is also the treatment of choice in patients who are pregnant or breastfeeding. Alternative regimens are available for patients who are penicillin allergic or decline IM therapy.
Complicated infection
Patients with PID or epididymo-orchitis should receive 1 gram of ceftriaxone IM as a single dose in addition to the regimen recommended by local antibiotic guidelines. Inpatient admission may be required for intravenous antibiotics. Local guidelines and specialist advice should always be sought.
Disseminated gonorrhoea
Patients initially require a course of parenteral treatment.
- Ceftriaxone 1 g IM or intravenously (IV) every 24 hours, OR
- Ciprofloxacin 500 mg IV every 12 hours (if susceptibility known)
Therapy should be continued for a total of seven days but in the presence of clinical improvement they may be stepped down to an oral regimen.
Co-infection with chlamydia
Patients should be treated in accordance with current guidelines for the treatment of chlamydia in addition to therapy for gonorrhoea
Complications
Gonorrhoea rarely causes meningitis and/or endocarditis from disseminated infection.
Men or transwomen
- Prostatitis
- Epididymo-orchitis
- Urethral stricture
- Infertility
Women or transmen
- Pelvic inflammatory disease
- Tubal infertility
- Peri-hepatitis (Fitz-Hugh-Curtis syndrome)
- Pregnancy complications: ectopic, miscarriage, fetal loss, congenital infections
Disseminated
- Tendinitis +/- tenosynovitis
- Septic arthritis
- Meningitis
- Endocarditis
Last updated: October 2024
Further reading
British Association for Sexual Health and HIV (BASHH) guidelines.
Have comments about these notes? Leave us feedback
